Prescription drug costs for public drug plans covering most of Canada increased 7.4 per cent in 2017/18 to reach $11.4 billion, according to a new report by the Patented Medicine Prices Review Board. It’s a significant increase compared to the more modest 1.9 per cent growth in prescription drug costs from 2016/17. The board looked at data […]

If the Canada-United States-Mexico trade agreement goes through it could have a significant impact on the cost of private drug plans. Currently on hold until members of Parliament return to Ottawa after the summer break, the agreement as written would extend the data protection term for biologic drugs to 10 years from the current eight. If […]

On May 22, the 2019 Calgary Benefits Summit focused on how employers can make their organization its healthiest by starting with their benefits plans. Here’s what you missed! How a Blue Zone concept can benefit the workplace What can a small Alberta town of 70,000 people do when the provincial government’s response to, “We need […]

British Columbia is expanding its use of biosimilars, effective November 2019. The move is expected to create opportunities for new drug listings and increase current coverage for patients, according to a press release from the B.C. government. The first two drugs to come into immediate effect are Jardiance, a diabetes drug, and Taltz, an arthritis drug. It will change the game considerably, says Suzanne Lepage, a private […]

Specialty medications and high-cost drugs for common conditions are driving up private plan drug spending, according to Express Scripts Canada’s annual drug trends report. The report found the rapid increases in drug spending for private plans over the last 20 years have started to slow. The average annual drug spending per plan member was up just 0.9 per cent […]

Health Canada has proposed amendments to the Food and Drug Regulations that would make it easier for drug companies to get authorization to produce generic versions of brand name drugs. The amendments would allow Health Canada to more quickly to approve generic drug applications that have the same active ingredients as their brand name equivalent […]

On the eve of his retirement, with more than 40 years in benefits under his belt, Aon’s Art Babcock considers the industry’s move to technology, health-care spending accounts and drone piloting Q. How did you get into the group benefits industry? A. I began my career in the insurance industry out of university, with Metropolitan […]
Though many plan sponsors are still using step therapy to manage drug plan costs, pharmacogenetic testing is receiving attention as more providers enter the market, lower the cost and familiarize employers and insurers with the technology. As it becomes more sophisticated, there’s more information about how pharmacogentic testing works, says Sandra Ventin, associate vice-president at […]

The Competition Bureau has discontinued an inquiry into allegations that Janssen Inc. inhibited the Canadian market for biosimilar products that compete with its biologic product Remicade. Given the significant impact of biologics in Canada, the report highlights some of the competition issues related to the biologics and biosimilars market. Although the bureau expressed concerns about the […]
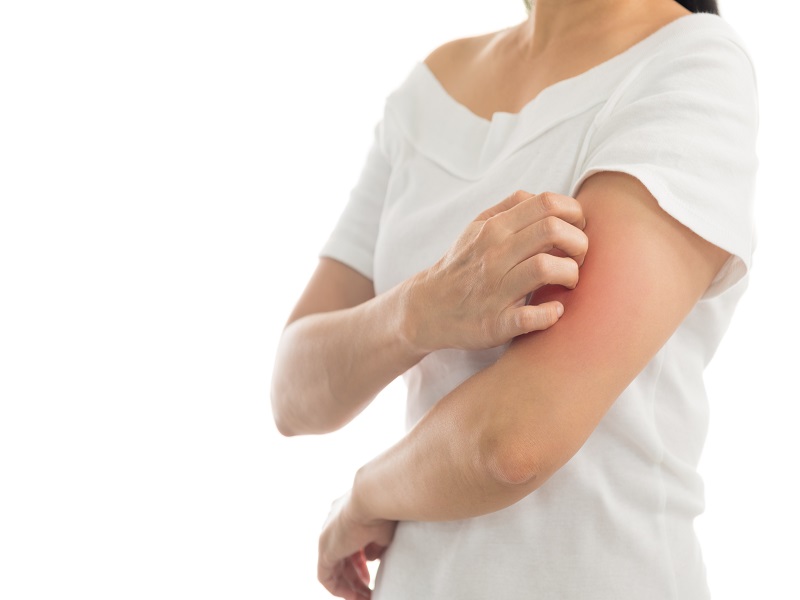
Minna Fein-Leenen was living a happy, healthy life until 2011 when she developed atopic dermatitis, a chronic inflammatory skin disease. “It was devastating. I thought I was losing my mind,” the Ottawa resident told delegates at the 2018 Calgary Drug Trends Summit on Oct. 25. “The intensity of the itching can only be described as […]